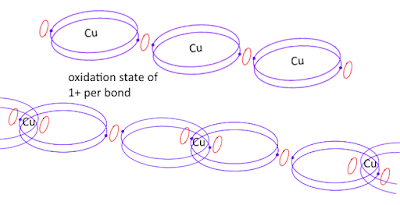
Both variations of the type of bonds \(Cu\) has in a metal lattice provides \(+1\) oxidation state per bond to the element when it is removed from the lattice. At the same time of its removal, if one electron is also released then the ions has \(2+\) oxidation state per bond broken.
Which means for metal to form crystal lattice charges must be removed from it during crystallization. The element has a oxidation state of \(1+\) in the lattice, due to two of its atoms sharing one electron per bond. When two bonds hold the atom in place then, two halves of each electron at each bond makes one electron per two bonds. When both bonds are broken the atom has one electron for two electron vacancies. This makes the element at an oxidation state of \(1+\) without loosing any electrons.
And they are all \(1+\) in the metal lattice.