But, what if the orbit is unpaired? Weak field moment is not zero as the revolving opposite particles do not generate the same weak field but weak fields that are orthogonal.
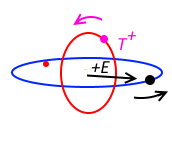
What if the orbit does not have an opposite particle? Does radiating weak field wave kills off the spin? No, the weak field wave generated by the single particle in orbit slows down an passing opposite particle on its approach to the orbit. As shown in the diagram below, in the plane parallel to the orbit, the opposite particle is slowed by the weak field as the result of Lorentz Force, \(F\). \(F\) acts to slow the tangential velocity \(v\). The opposite particle will be given the same spin as the particle in orbit. This spinning opposite particle generates a weak field opposite to the particle in orbit and is slowed in the direction along the weak field on its approach to the orbit.
This is how the orbiting particle proactively attracts an opposite spinning particle. It slows the particle down. This opposite particle is captured by the opposite end of the weak field (generated by a \(T^{+}\) particle) holding the other particle on the orbit.
When both particles are in orbit, they spin as a dipole. Both generate a weak field that is orthogonal to each other, the weak fields do not cancel.
What motivate covalent bond formation then? When the opposite particle is removed (orbit ionized), instead of attracting an opposite particle, the weak field attracts the field weak of another orbit. As the weak fields merge both orbiting particles are spinning in the same sense and are repelled by each other to across the diameter of the orbit.
These paired orbits then attracts two opposite particles in the manner as described above.
We have a covalent bond, but still both unpaired orbits that form the bond must first be ionized (ie. opposite particle removed) before being paired. The opposite particles are attracted back to the orbits after the orbits paired up.
So, a covalent bond requires double the ionization energy of a unpaired orbit to initiate.
Good night.